What Makes This Plant Derived Absorbent… One of the Best Absorbent In The World? … Kepler® Absorbs Anything And Everything In Liquid Form And Encapsulates.
All About Polarity…
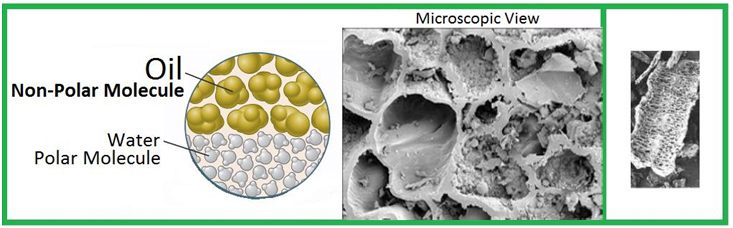
Water is a polar molecule and this is a key reason why water and oil do not mix. Wondering what polarity is? Polarity is when one end is positively charged while the other end is negatively charged. Each water molecule is made of two hydrogen atoms and one oxygen atom. But the atoms are not arranged in a line. The two hydrogen atoms cling to one side of the oxygen atom making the molecule look something like a Mickey Mouse head. The electrons in the molecule spend more time on the oxygen side of the molecule, giving this side a negative charge and the hydrogen side a positive charge. Only other polar molecules can dissolve in water because polar molecules dissolve only in polar solvents and non-polar molecules dissolve only in non-polar solvents.
Oil and water don’t mix because oil is made up of non-polar molecules while water molecules are polar in nature. Because water molecules are electrically charged, they get attracted to other water molecules and exclude the oil molecules. This eventually causes the oil molecules, or lipids, to clump together.
Oil which is non-polar displaces water absorbed by Kepler®. Oil can displace water, but water cannot displace oil out of Kepler®. Therefore, there is no oil film. Oil is locked in and “encapsulated”.